Example Of Solvation . A sodium ion solvated by water, from wikimedia commons. solvation is the process by which solvent molecules surround and interact with solute ions or molecules. solvation, or dissolution, is the process by which a solute dissolves into a solvent. solvation is the process in which molecules of a solvent attract the particles of a solute. An important specific example of solvation is hydration,. explain how the solvation process describes the dissolution of a solute in a solvent. The main forces in solvation are ion. A solvated ion or molecule is surrounded by solvent. the interactions between the solute particles and the solvent molecules is called solvation. As indicated in figure 13.2.1, solvation.
from www.dreamstime.com
explain how the solvation process describes the dissolution of a solute in a solvent. The main forces in solvation are ion. the interactions between the solute particles and the solvent molecules is called solvation. A solvated ion or molecule is surrounded by solvent. An important specific example of solvation is hydration,. solvation, or dissolution, is the process by which a solute dissolves into a solvent. solvation is the process in which molecules of a solvent attract the particles of a solute. A sodium ion solvated by water, from wikimedia commons. solvation is the process by which solvent molecules surround and interact with solute ions or molecules. As indicated in figure 13.2.1, solvation.
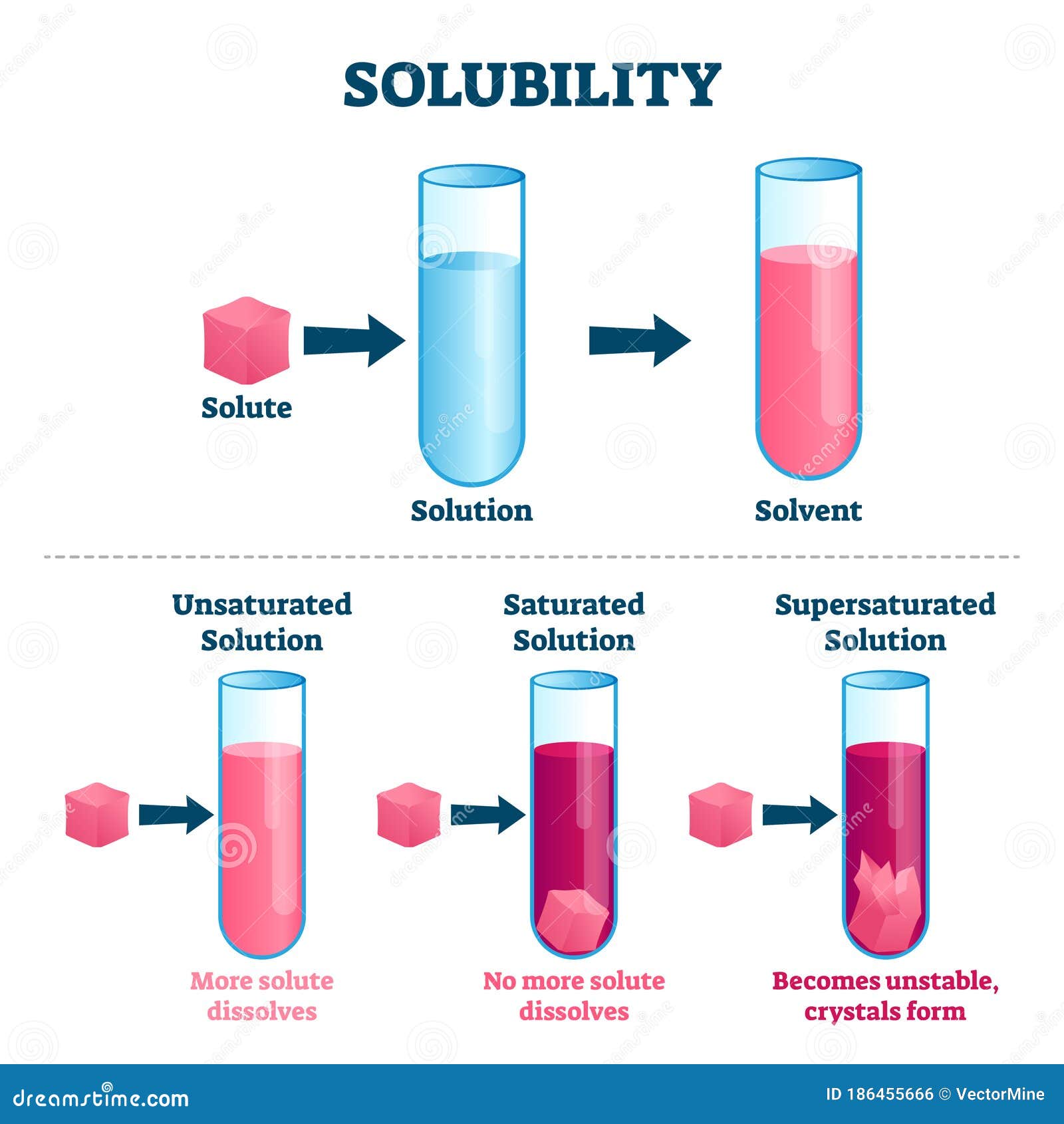
Solubility Vector Illustration. Labeled Solute, Solvent and Solution
Example Of Solvation A sodium ion solvated by water, from wikimedia commons. the interactions between the solute particles and the solvent molecules is called solvation. A sodium ion solvated by water, from wikimedia commons. As indicated in figure 13.2.1, solvation. solvation, or dissolution, is the process by which a solute dissolves into a solvent. An important specific example of solvation is hydration,. explain how the solvation process describes the dissolution of a solute in a solvent. solvation is the process by which solvent molecules surround and interact with solute ions or molecules. A solvated ion or molecule is surrounded by solvent. The main forces in solvation are ion. solvation is the process in which molecules of a solvent attract the particles of a solute.
From animalia-life.club
Example Of Solution In Chemistry Example Of Solvation A solvated ion or molecule is surrounded by solvent. As indicated in figure 13.2.1, solvation. A sodium ion solvated by water, from wikimedia commons. explain how the solvation process describes the dissolution of a solute in a solvent. An important specific example of solvation is hydration,. solvation is the process in which molecules of a solvent attract the. Example Of Solvation.
From surfguppy.com
Solubility Surfguppy Chemistry made easy for visual learners Example Of Solvation solvation is the process by which solvent molecules surround and interact with solute ions or molecules. An important specific example of solvation is hydration,. A sodium ion solvated by water, from wikimedia commons. The main forces in solvation are ion. solvation is the process in which molecules of a solvent attract the particles of a solute. solvation,. Example Of Solvation.
From www.dorthonion.com
Solubility Scholars Online Chemistry Example Of Solvation An important specific example of solvation is hydration,. As indicated in figure 13.2.1, solvation. A sodium ion solvated by water, from wikimedia commons. explain how the solvation process describes the dissolution of a solute in a solvent. solvation is the process in which molecules of a solvent attract the particles of a solute. solvation is the process. Example Of Solvation.
From www.slideshare.net
the solvation process Example Of Solvation A solvated ion or molecule is surrounded by solvent. solvation is the process by which solvent molecules surround and interact with solute ions or molecules. A sodium ion solvated by water, from wikimedia commons. the interactions between the solute particles and the solvent molecules is called solvation. An important specific example of solvation is hydration,. The main forces. Example Of Solvation.
From www.albert.io
Solubility Rules The Ultimate Guide to AP® Chemistry Albert.io Example Of Solvation The main forces in solvation are ion. As indicated in figure 13.2.1, solvation. An important specific example of solvation is hydration,. solvation is the process in which molecules of a solvent attract the particles of a solute. A sodium ion solvated by water, from wikimedia commons. A solvated ion or molecule is surrounded by solvent. solvation is the. Example Of Solvation.
From www.slideserve.com
PPT Ch. 14 Mixtures & Solutions PowerPoint Presentation, free Example Of Solvation the interactions between the solute particles and the solvent molecules is called solvation. The main forces in solvation are ion. An important specific example of solvation is hydration,. A solvated ion or molecule is surrounded by solvent. solvation, or dissolution, is the process by which a solute dissolves into a solvent. A sodium ion solvated by water, from. Example Of Solvation.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Example Of Solvation solvation is the process in which molecules of a solvent attract the particles of a solute. A solvated ion or molecule is surrounded by solvent. A sodium ion solvated by water, from wikimedia commons. solvation, or dissolution, is the process by which a solute dissolves into a solvent. An important specific example of solvation is hydration,. the. Example Of Solvation.
From www.youtube.com
Factors that Affect Solubility CLEAR & SIMPLE YouTube Example Of Solvation The main forces in solvation are ion. explain how the solvation process describes the dissolution of a solute in a solvent. the interactions between the solute particles and the solvent molecules is called solvation. solvation is the process by which solvent molecules surround and interact with solute ions or molecules. An important specific example of solvation is. Example Of Solvation.
From surfguppy.com
Solubility Surfguppy Chemistry made easy visual learning Example Of Solvation solvation, or dissolution, is the process by which a solute dissolves into a solvent. the interactions between the solute particles and the solvent molecules is called solvation. As indicated in figure 13.2.1, solvation. The main forces in solvation are ion. solvation is the process by which solvent molecules surround and interact with solute ions or molecules. . Example Of Solvation.
From www.slideserve.com
PPT Solvation Models PowerPoint Presentation, free download ID927911 Example Of Solvation solvation is the process by which solvent molecules surround and interact with solute ions or molecules. explain how the solvation process describes the dissolution of a solute in a solvent. the interactions between the solute particles and the solvent molecules is called solvation. The main forces in solvation are ion. An important specific example of solvation is. Example Of Solvation.
From www.slideserve.com
PPT Solvation Models PowerPoint Presentation, free download ID927911 Example Of Solvation explain how the solvation process describes the dissolution of a solute in a solvent. the interactions between the solute particles and the solvent molecules is called solvation. An important specific example of solvation is hydration,. A solvated ion or molecule is surrounded by solvent. solvation is the process by which solvent molecules surround and interact with solute. Example Of Solvation.
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Example Of Solvation explain how the solvation process describes the dissolution of a solute in a solvent. A solvated ion or molecule is surrounded by solvent. A sodium ion solvated by water, from wikimedia commons. As indicated in figure 13.2.1, solvation. The main forces in solvation are ion. solvation is the process in which molecules of a solvent attract the particles. Example Of Solvation.
From www.differencebetween.com
Difference Between Solvation Energy and Lattice Energy Compare the Example Of Solvation A sodium ion solvated by water, from wikimedia commons. explain how the solvation process describes the dissolution of a solute in a solvent. The main forces in solvation are ion. the interactions between the solute particles and the solvent molecules is called solvation. A solvated ion or molecule is surrounded by solvent. As indicated in figure 13.2.1, solvation.. Example Of Solvation.
From www.slideserve.com
PPT Types of Chemical Reactions & Solutions PowerPoint Presentation Example Of Solvation solvation is the process by which solvent molecules surround and interact with solute ions or molecules. A sodium ion solvated by water, from wikimedia commons. The main forces in solvation are ion. the interactions between the solute particles and the solvent molecules is called solvation. As indicated in figure 13.2.1, solvation. An important specific example of solvation is. Example Of Solvation.
From www.youtube.com
General Chemistry Properties of Liquids, Solids, & Solutions Example Of Solvation the interactions between the solute particles and the solvent molecules is called solvation. solvation is the process by which solvent molecules surround and interact with solute ions or molecules. solvation, or dissolution, is the process by which a solute dissolves into a solvent. explain how the solvation process describes the dissolution of a solute in a. Example Of Solvation.
From www.slideserve.com
PPT Solutions in Chemistry PowerPoint Presentation, free download Example Of Solvation As indicated in figure 13.2.1, solvation. The main forces in solvation are ion. A solvated ion or molecule is surrounded by solvent. An important specific example of solvation is hydration,. explain how the solvation process describes the dissolution of a solute in a solvent. solvation is the process in which molecules of a solvent attract the particles of. Example Of Solvation.
From www.youtube.com
Solvation and hydration / best chemistry solution YouTube Example Of Solvation the interactions between the solute particles and the solvent molecules is called solvation. A solvated ion or molecule is surrounded by solvent. As indicated in figure 13.2.1, solvation. solvation, or dissolution, is the process by which a solute dissolves into a solvent. solvation is the process by which solvent molecules surround and interact with solute ions or. Example Of Solvation.
From www.youtube.com
Chemistry 14.3 Factors Affecting Solvation (part 1) YouTube Example Of Solvation the interactions between the solute particles and the solvent molecules is called solvation. solvation is the process in which molecules of a solvent attract the particles of a solute. The main forces in solvation are ion. An important specific example of solvation is hydration,. As indicated in figure 13.2.1, solvation. A sodium ion solvated by water, from wikimedia. Example Of Solvation.